Saltwater batteries are not similar to lithium-ion batteries or lead acid batteries for use in portable devices or any automobiles. This is because they can’t hold as much charge in the same size and weight, or putting it another way, they are less energy-dense. But they hold some powerful advantages in applications in which size and weight are less important.
The electrolyte for a saltwater battery is nothing more than — salt water — hence the device’s name comes from it. The anode can be carbon, and the cathode can be a material such as manganese oxide. (Or anode can be copper and cathode can be zinc or aluminum).
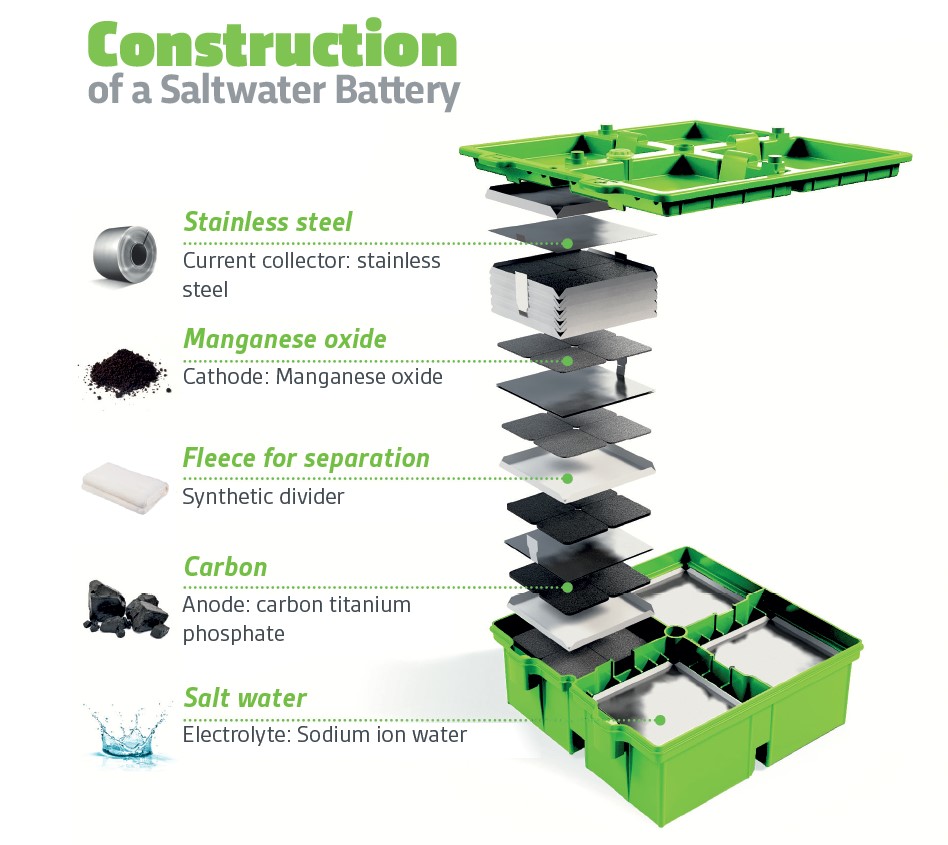
With no hazardous materials in their construction, unlike lithium-ion batteries, they are non-toxic and they cannot explode. There is no need for the complex and troublesome electronic circuitry that every lithium-ion battery needs to ensure that they charge and discharge only within safe parameters. In addition, unlike their lithium-based cousins, saltwater batteries can be more deeply discharged (drained of electrical energy) with no damage to the battery.
The biggest disadvantage of all forms of salt-water batteries is that, to store a given amount of electricity, they are bulkier and heavier than other commercially available batteries. As it turns out, there is one huge potential use for these devices in which that will be no issue at all — smoothing out power from large-scale renewable-energy-generation plants.
